Where Should Schedule Ii Prescriptions Be Filed
Memoranda of oral prescriptions are required to be kept on file by the owner of the pharmacy for not less than two years. The written prescription referred to in 541-3408 shall be written with ink or individually typed or printed.
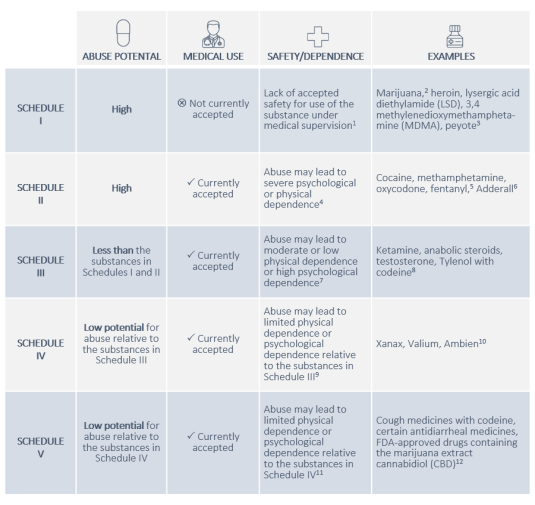
The Controlled Substances Act Csa A Legal Overview For The 117th Congress Everycrsreport Com
External drugs separate from internal drugs a.

. The original paper prescription must be maintained in the patient file. Schedule II prescriptions for individuals in a hospice or a long-term care facility can be transmitted by fax by a delegated agent. Drug carts should not be stored in the hall 2.
There are some rare circumstances epidemic unforeseen accident etc under which a practitioner may issue a valid oral prescription for a Schedule II controlled substance. Code 20-2-58 2008. It should be noted that allowance for faxing prescriptions for schedule II controlled substances is not permissible as a general rule in non-LTCF settings.
Schedule II controlled Food Drug. In a letter to the New Mexico Regulation and Licensing Department Board of Pharmacy in January 2010 the DEA acknowledged that interpreting this position to mean that absent the ability to make oral changes a Schedule II prescription must be returned to the prescribing practitioner for correction or reissuance creates a significant issue that they are. Prescriptions for controlled substances other than Schedule IIs are valid.
2 In addition there is no federal time limit on when a Schedule II prescription must be filled after being signed by a prescriber. Key with the charge nurse c. If authorized by DEA other records pertaining to Schedules II through V drugs such as invoices may be maintained in an off-site database or in secured storage.
Prescriptions shall be retained in conformity with the requirements of RCW 6950306. Prescriptions for Schedule II medications may not be refilled. Pharmacy regulations permit Pharmacy.
There may be variations in CSA schedules between individual states. Twenty or thirty years ago most facilities simply kept stock narcotics in a locked cabinet in a locked. The Registrant should make a list of the unique form numbers and record the date when each is used.
Doctors May Now Electronically Prescribe Schedule II Drugs. A prescription for a Schedule II drug must be handwritten or typed and must be signed by the prescribing physician in the same way that he would sign a check or legal document full name. The prescription shall contain the name address and telephone number of the prescriber.
Can I add the Earliest Fill Date to the prescription. A prescription for a controlled substance other than one controlled in. Section 2003 of the SUPPORT Act mandates that the prescribing of a Schedule II III IV or V controlled substance under Medicare Part D should be done electronically in accordance with an electronic prescription drug program beginning 2021 subject to any exceptions which the Department of Health and Human Services HHS may specify.
The Controlled Substances Act CSA schedule information displayed applies to substances regulated under federal law. Schedule II double locked standard of practice all controls double locked b. Both exceptions are effective on March 16 2020 and will last through the duration of the public health.
According to Texas Health and Safety Code 481074d-1 practitioners have the option of issuing. Schedule II through V drugs must be handled as controlled substances and securely locked usually with double locks or special locks in a substantially constructed cabinet. 1 A sample Form.
The DEA also noted that emergency oral prescriptions for Schedule II drugs should be limited to the amount adequate to treat the patient during the emergency period. An official prescription written for a Schedule II controlled substances must be filled within 30 days after the date the prescription was issued. If you are in doubt about the refilling of a.
Pharmacies may install at a LTCF but in no other setting an automated dispensing system ADS. A new written prescription is required if your physician wants you to continue to take a Schedule II medication after completion of the first prescription. Substances require a written prescription unless very limited exceptions apply eg emergency or for resident of long-term care facility.
C In emergency situations as defined by rule of the commission a substance included in Schedule II may be dispensed upon oral prescription of a practitioner reduced promptly to writing and filed by the pharmacy. If a pharmacy orders Schedule 2 drugs it must first fill out the DEA Form 222. When the Drug Enforcement Administration DEA grants the Registrant permission to use Schedule I or II C-I or C-II drugs they will send DEA Form 222 order formsThe Registrant is responsible for securing the Forms 222 and retaining the executed and unexecuted forms.
Methadone is a C-II controlled substance that is commonly used for Opioid addiction When prescriptions for controlled substances in C-III through C-V will be filed with other non-controlled drug prescriptions the controlled drug prescriptions are designated in the pharmacy by stamping with a C that must appear. Schedule I and II controlled substances may be ordered by filling out a Drug Enforcement Agency DEA Form 222 or by electronically completing the DEA Controlled Substance Ordering System CSOS. All executed order forms prescriptions and inventories of Schedules II through V drugs shall be maintained at the same address as the stock of drugs to which the records pertain.
The following drugs are listed as Schedule 2 II Drugs by the Controlled Substances Act CSA. No Aides CNAs in med room unless nurse present d. Emergency oral prescriptions can be transmitted only by a prescriber and must be followed up by a written prescription within seven days.
Schedule II prescriptions must be presented to the pharmacy in written form and signed by the prescriber. 1 There are no federal quantity limits on Schedule II prescriptions. All drugs and non-Rx drugs must be locked a.
An in-depth look into the past present and future of the electronic prescribing of controlled substances. On this form the pharmacy must document the number and size of packages and the name of the drug being ordered. We have had many inquiries from pain management doctors wanting details of the Drug Enforcement Agencys DEA lift of the ban on ePrescribing Schedule II drugs.
Prescriptions for both legend drugs and controlled substances to be transmitted. That being said the pharmacist must ensure that the controlled. Oral prescriptions for Schedule II drugs are not permitted.

The Federal Controlled Substances Act A Primer For Providers

The Federal Controlled Substances Act A Primer For Providers

The Federal Controlled Substances Act A Primer For Providers
0 Response to "Where Should Schedule Ii Prescriptions Be Filed"
Post a Comment